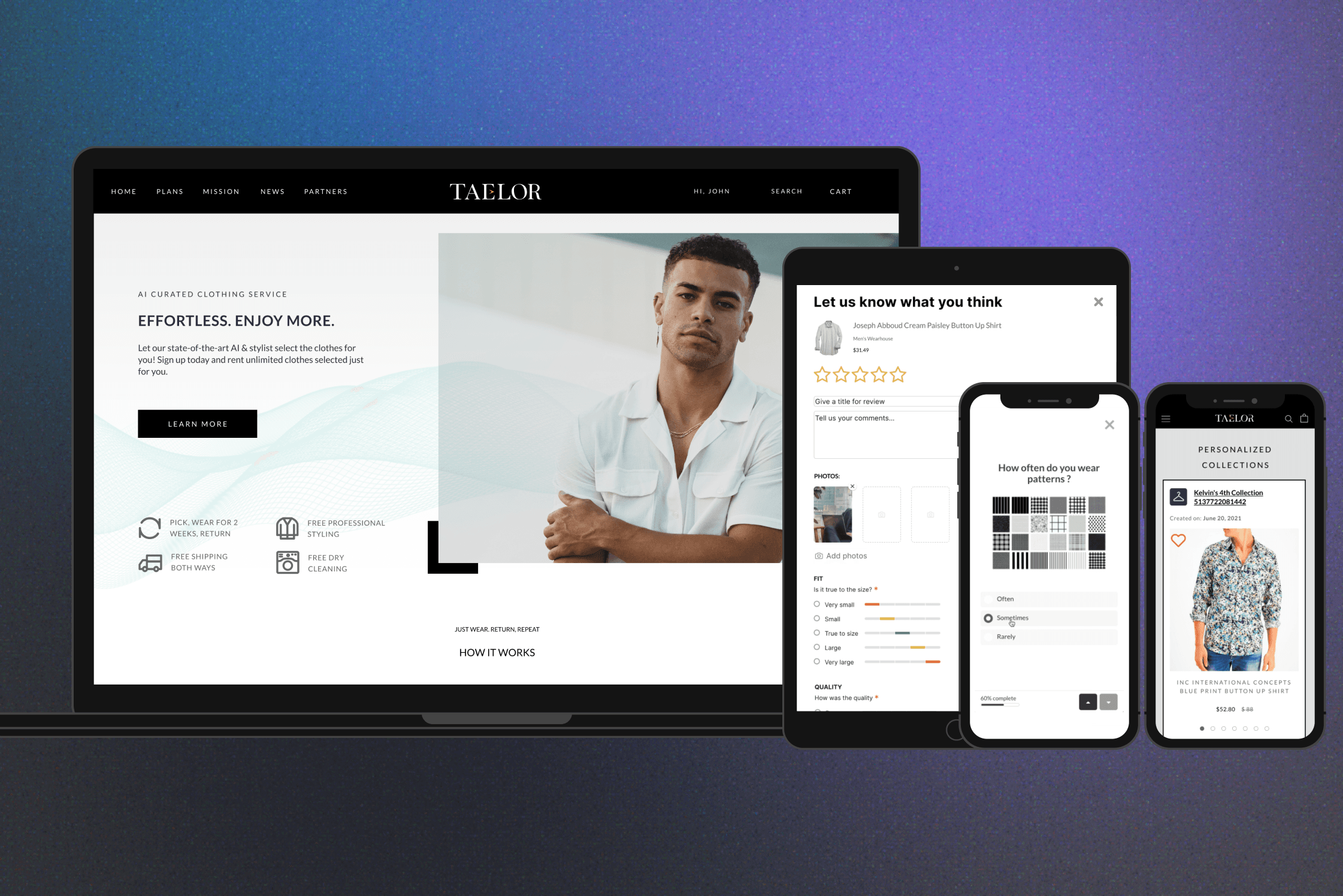
Designing for insights exploratory: An AI-driven Synthetic Data Platform for Clinical Research
Strategy
Strategy
Strategy
Product Design
Product Design
Product Design
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!



overview
Simulants is an AI-driven synthetic data solution that transforms de-identified patient-level data into high-fidelity datasets using a validated, patented algorithm. I led the design of both the internal synthetic data generation tool and the external exploration platform, collaborating with a cross-functional team to evolve this proven technology into a scalable SaaS product—enabling secure, efficient data exploration, analysis, and collaboration across teams and clients.
Outcome
The platform was launched in April 2024 enabled clients to explore synthetic clinical trial data derived from 30,000+ studies and 9 million patients, unlocking cross-sponsor insights while protecting patient privacy and intellectual property. Clients reported that the solution streamlined data exploration and collaboration, helping sponsors and researchers identify safety signals earlier and refine clinical trial protocols with greater confidence.
Timeline
Aug 2023 - Aug 2024. Multiple releases
Industry
Healthcare / Life Science
B2B SaaS
My Responsibility
UX strategies and architect
Discovery research
UI and interaction Design
Usability testing

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”
More Projects
Designing for insights exploratory: An AI-driven Synthetic Data Platform for Clinical Research
Strategy
Strategy
Strategy
Product Design
Product Design
Product Design
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!



overview
Simulants is an AI-driven synthetic data solution that transforms de-identified patient-level data into high-fidelity datasets using a validated, patented algorithm. I led the design of both the internal synthetic data generation tool and the external exploration platform, collaborating with a cross-functional team to evolve this proven technology into a scalable SaaS product—enabling secure, efficient data exploration, analysis, and collaboration across teams and clients.
Outcome
The platform was launched in April 2024 enabled clients to explore synthetic clinical trial data derived from 30,000+ studies and 9 million patients, unlocking cross-sponsor insights while protecting patient privacy and intellectual property. Clients reported that the solution streamlined data exploration and collaboration, helping sponsors and researchers identify safety signals earlier and refine clinical trial protocols with greater confidence.
Timeline
Aug 2023 - Aug 2024. Multiple releases
Industry
Healthcare / Life Science
B2B SaaS
My Responsibility
UX strategies and architect
Discovery research
UI and interaction Design
Usability testing

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”
More Projects
Designing for insights exploratory: An AI-driven Synthetic Data Platform for Clinical Research
Strategy
Strategy
Strategy
Product Design
Product Design
Product Design
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!
Due to NDA restrictions, this case study highlights only publicly available information. If you’d like to learn more about my role in the process and design impact, please reach out, let’s connect!



overview
Simulants is an AI-driven synthetic data solution that transforms de-identified patient-level data into high-fidelity datasets using a validated, patented algorithm. I led the design of both the internal synthetic data generation tool and the external exploration platform, collaborating with a cross-functional team to evolve this proven technology into a scalable SaaS product—enabling secure, efficient data exploration, analysis, and collaboration across teams and clients.
Outcome
The platform was launched in April 2024 enabled clients to explore synthetic clinical trial data derived from 30,000+ studies and 9 million patients, unlocking cross-sponsor insights while protecting patient privacy and intellectual property. Clients reported that the solution streamlined data exploration and collaboration, helping sponsors and researchers identify safety signals earlier and refine clinical trial protocols with greater confidence.
Timeline
Aug 2023 - Aug 2024. Multiple releases
Industry
Healthcare / Life Science
B2B SaaS
My Responsibility
UX strategies and architect
Discovery research
UI and interaction Design
Usability testing

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”

“... the sponsor says has helped with designing and executing faster, safer trials that lower patient burden, reduce costs and deliver promising and effective therapeutics to patients. The judges noted that AI Simulants were able to shorten clinical trial timelines up to two years (saving $10m-$15m) and determine optimal regimens and study design by accessing global historical data…”